Medical Device Software Testing Tools

General validation principles of medical device software or the validation of software used to design develop or manufacture medical devices.
Medical device software testing tools. Such processes ensure effective performance accurate reading and safety of devices. With matlab and simulink you can. A medical device testing strategy must incorporate compliance processes and technical testing strategies for better performance and effectiveness of medical devices. Manufacturers that have embedded software in medical devices need to follow robust software development and testing processes to capture all information necessary for compliance.
Manufacturers need to have a strong testing strategy in place right from the design stage as performing an exhaustive testing of a produced device is ineffective and inefficient. Develop and test advanced algorithms and entire systems before implementation. Learn how the right toolset makes medical device compliance easy. Matlab and simulink enable engineers to speed up medical device software and hardware development by efficiently integrating and automating the various phases of design implementation and verification.
Simulate and test embedded software alongside mechatronic systems early. And that calls for medical device development tools. We have a unique understanding of the complexity of medical devices due to the criticality and integrated ecosystem of hardware and software. The fda s medical device development tools mddt program is a way for the fda to qualify tools that medical device sponsors can use in the development and evaluation of medical devices.
Our quality solutions have supported dozens of leading brands in fact medical devices make up our largest us sector. Medical device testing needs to be thorough. Failure proof your medical device with expert testing. Vectorcast by vector is the solution for fda compliant and iec 62304 compliant medical embedded application testing.
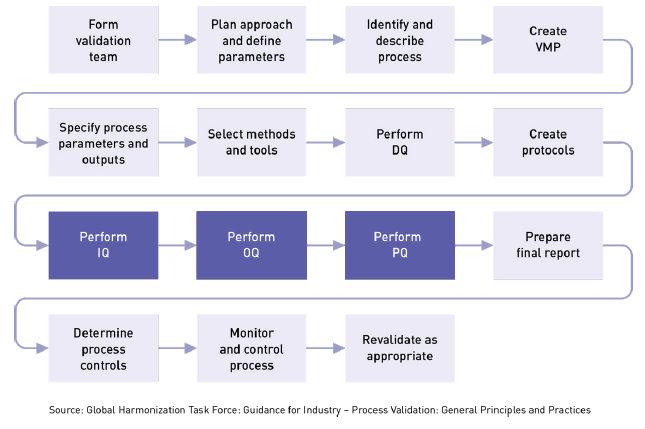
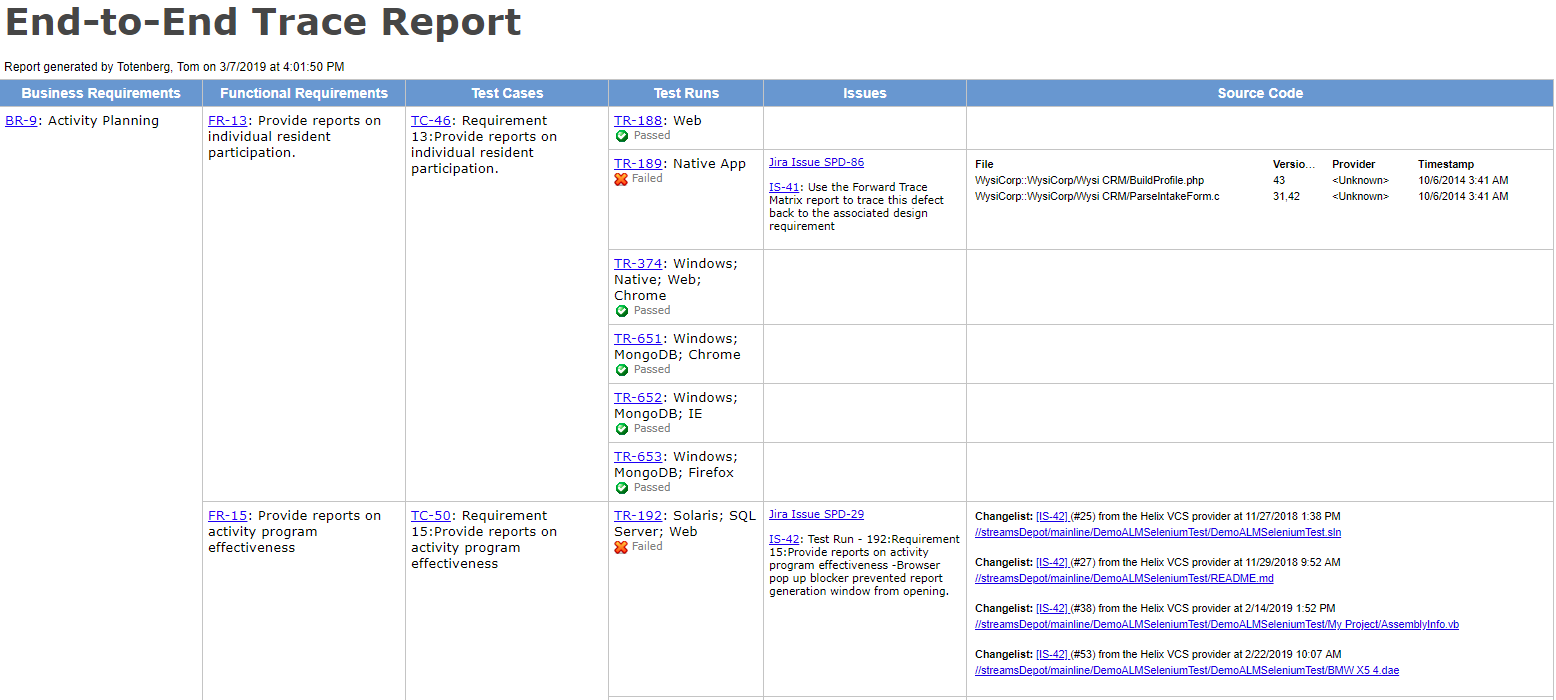
Iec 62304 medical device software software life cycle processes specifies life cycle requirements for the development to medical software and software within medical devices. Iec 62304 is applicable to all software for medical devices and applications and covers the processes and activities around the production of embedded and free standing software. Software testing is a critical part of ensuring the performance of medical devices to pass fda audits and support the guidelines defined in iec 62304. Medical device development teams are under pressure.



%20Design%20Verification%20%26%20Validation.png?width=1700&name=(cover)%20Design%20Verification%20%26%20Validation.png)








































