Medical Device Gamma Sterilization
To validate the sterilization dose an accurate measurement of radiation exposure is crucial.
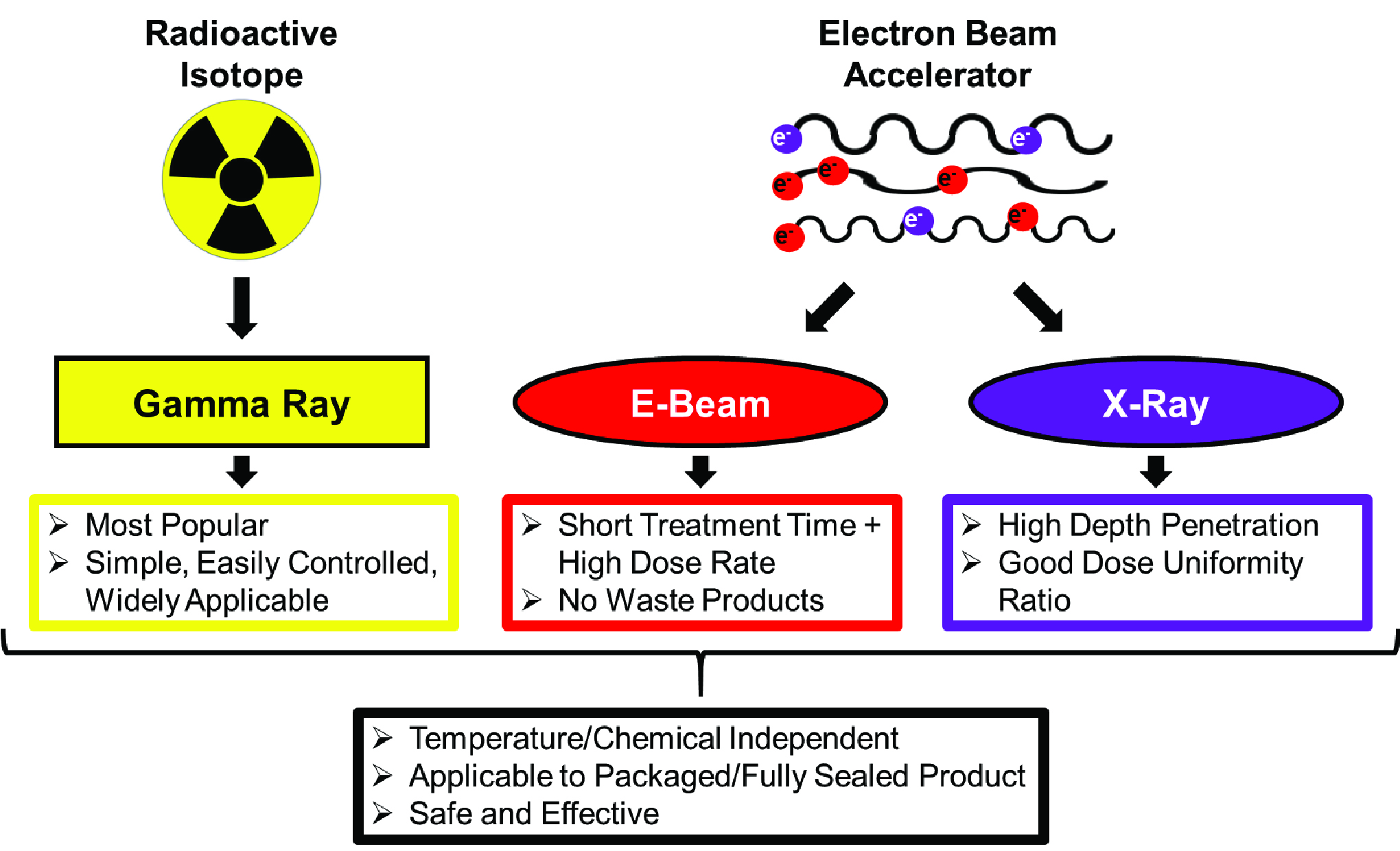
Medical device gamma sterilization. Eto gamma steam shelf life validation 275 00 255 00 this live webinar course will provides guidance on medical device sterilization processes. These gamma rays can penetrate through the entire product deactivating whatever microorganisms may be present. The gamma irradiation process uses cobalt 60 radiation for a variety of applications including sterilization decontamination and materials modification. The gamma sterilization process deploys gamma radiation in the form of cobalt 60 and an electron.
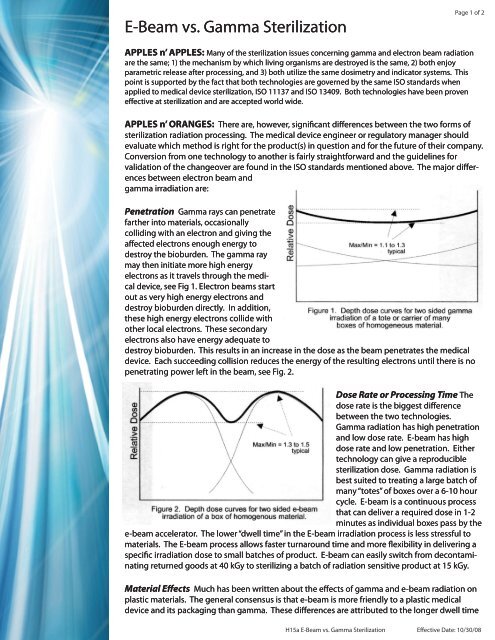
Gamma photons have the ability to penetrate deeply into material to provide a uniform sterilization dose throughout the volume of material. Gamma irradiation sterilization is performed by exposing the product to a radiation source typically cobalt 60 isotope which decomposes into nickel 60 isotope firing off gamma rays in the process. Gamma irradiation offers good penetration of dense products and is ideal for many types of materials and their packaging. The pcl lab frequently sees medical devices that have been subjected to gamma irradiation sterilization.
Gamma irradiation is a simple and proven process for the safe reliable and effective sterilization of devices. Medical device sterilization process. Steris applied sterilization technologies ast offers the medical device industry s most comprehensive array of contract sterilization options using gamma irradiation electron beam irradiation x ray irradiation and ethylene oxide sterilization. It is an effective sterilization method due to its.
In fact it has been a go to standard sterilization method for most medical devices over the past 40 years. With a wealth of experience providing sterilization for medical devices steris ast has been providing a first. A list of recognized sterilization standards appears at fda s center for devices and radiological health cdrh s web site. To achieve your sterilization goals you may require ethylene oxide eo or eto gamma electron beam e beam x ray or nitrogen dioxide no2 technologies.
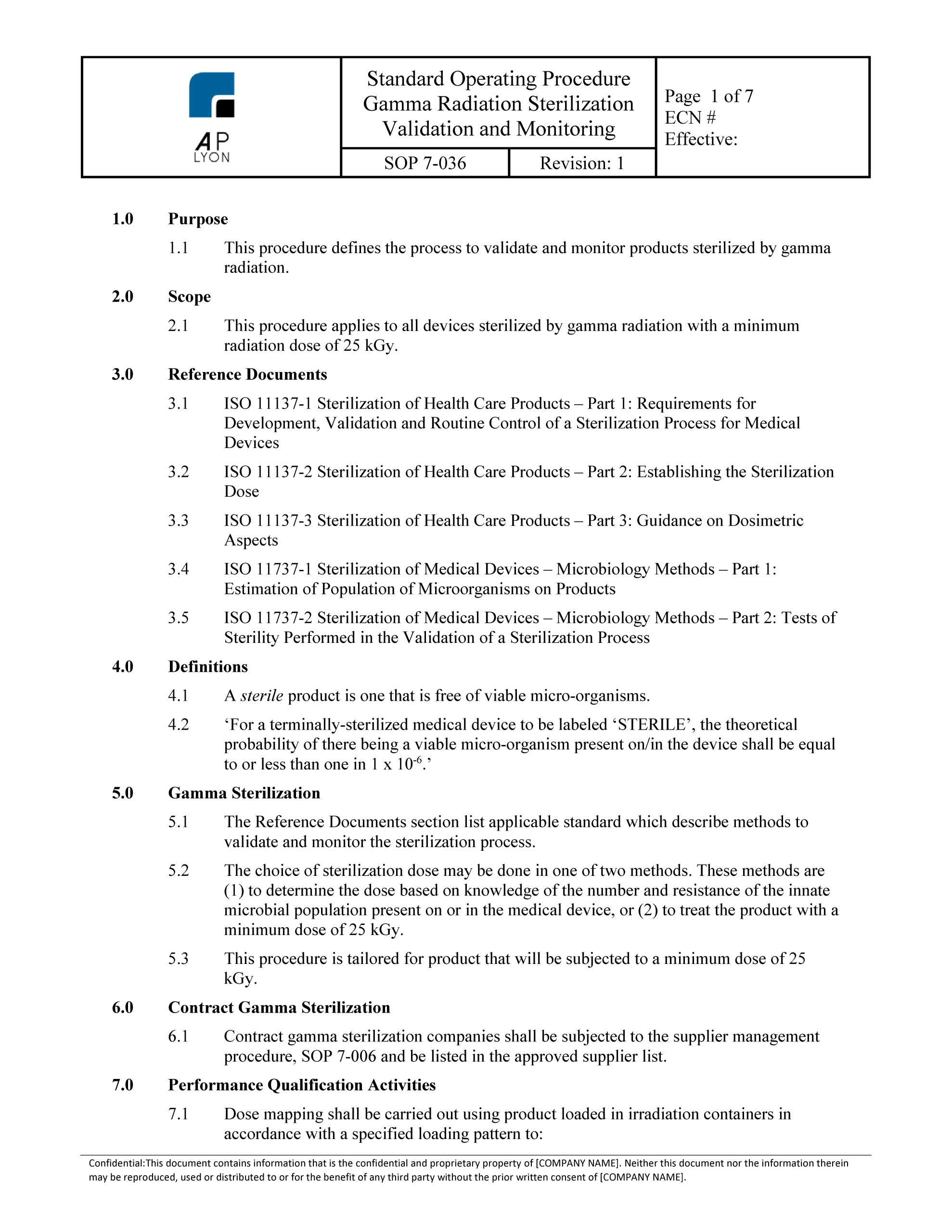
Sterility assurance treatment efficacy consistently meets product and regulatory requirements. Sys 047 gamma sterilization validation procedure the purpose of this procedure is to define the requirements for gamma sterilization validation and revalidation using the vd max25 method which has been outsourced by your company to a contract sterilizer. Firms may elect to comply with these standards. Safety offers proven track record in worker and product safety.